Our Process
Initial review typically takes at least 4 weeks from date of submission. Applicants may be asked to provide further information and clarification following the review.
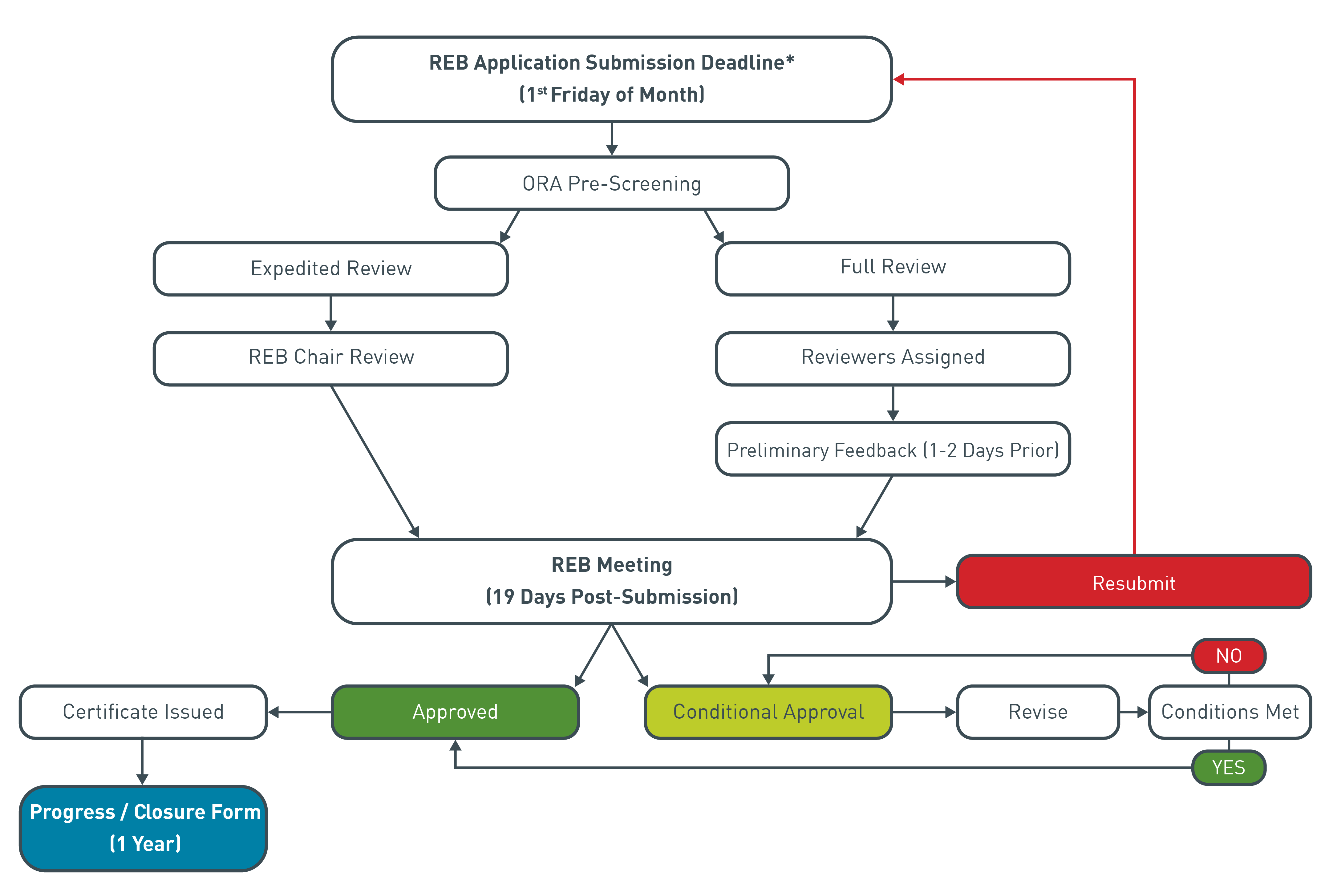
The following chart illustrates the Research Ethics Board process.
Notes for Investigators
- All CMCC investigators on an application to the Research Ethics Board must have updated research ethics training from the TCPS2CORE. This is a free course that can be accessed here.
- The Contact Investigator listed on the application will be invited to attend the meeting at which the application is reviewed; however, attendance is not required.
- Responses to reviewer comments and revisions for conditionally approved projects can be submitted at any time. The original reviewers will evaluate the responses and revisions. Applicants can typically expect a response in 1-2 weeks after submitting the revisions.
- Projects are rejected after three unsuccessful submissions.
- Amendments to an approved project should be submitted to the Office of Research Administration using the Amendment Form here.
- Investigators are required to keep the project certificate of approval on file. The certificate contains:
- The Project Number assigned when a proposal is initially submitted to the ORA;
- An REB Approval Number assigned when the project received ethical approval; and,
- The duration of approval.
- Investigators must report on the status of their project, using the Project Report or Closure form. Projects for which the ORA has not received a status report, and the expiry date has passed, will be deemed closed and will no longer be considered approved to continue testing.
Applicant Resources
Tri-Council Policy Statement
TCPS2CORE
CIHR patient partner materials
CMCC REB forms and materials
